
You may already know what electroplating is but what you may not know is how it works. Electroplating can be described as a common metal finishing or improving process used in a number of industrial applications.
It’s not new though as the earliest form, which became the modern electroplating process, occurred in the early 19th century. At this time, the development of bigger electric generators meant higher currents which drastically improved the process. With the advancement of industrial and manufacturing practices over the past two hundred years, this process has greatly evolved.
How does electroplating work?
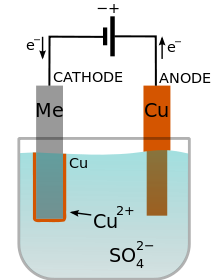
Electroplating uses an electric current to reduce dissolved metal cations so that they form a thin metal coating on an electrode. It’s known as electrodeposition because the process involves depositing a thin layer of metal onto the surface of a work piece referred to as the substrate.
The anode is connected to the positive terminal and the cathode (metal to be plated) is connected to the negative terminal. Both are immersed in a solution that contains an electrolyte and connected to an external supply of direct current. When DC power is applied, the anode is oxidized.
Metal atoms dissolve in the electrolyte solution and ions are reduced at the cathode forming a coating. The current through the circuit must be adjusted so that the rate of the anode being dissolved equals the rate at which the cathode is plated.
Different metals are coated using electroplating and preparing the right electrolyte is vital to plating quality. Commonly a single metallic element, not an alloy although some alloys like brass and solder can be electro-deposited. The ability to cover plating uniformly is called throwing power. The better the “throwing power” the more uniform the coating.
Applications of electroplating
Electroplating is widely used in numerous sectors for coating metal objects with a thin layer of a different metal. The added metal has a desired property the original object lacks and is primarily used to improve corrosion resistance. Chromium plating is a great example. So much so that you’ll see it on many objects such as car parts, bath taps, gas burners, wheel rims and many others.
Other uses include:
- Increase wear resistance
- Protect against surface abrasions
- Reduce friction
- Improve electrical conductivity (copper layer onto an electrical component)
- Prepare surfaces for better adhesion before painting or re-coating
Common metals used in electroplating include zinc, copper and tin but also precious metals like gold, silver and palladium. Plating is possible using single metals or with various combinations (alloys) that can provide additional value to the electroplating process. Silver and Tin are essential finishes for components that are designed to carry heavy electrical currents. A perfect example of this is Busbars.
There is no better way of controlling the quality of the finish other than doing it yourself. PRV Engineering invested in a silver and tin plating facility which has enabled them to do just that. We manufacture products which require numerous skills and processes. Finishing is the final operation and the quality and standard is of the utmost importance.
Get in touch if you need help with your project or if you have any questions about the available plating finishes, busbars or any of our other products and services.
This site uses Akismet to reduce spam. Learn how your comment data is processed.


 Mail:
Mail: 




Leave a Comments